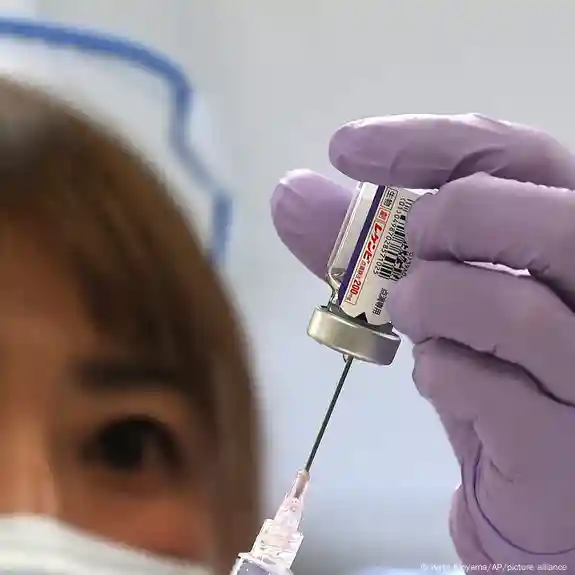
The antibody lecanemab is the first authorized therapy in Europe that targetsthe underlying process of Alzheimer’s, rather than only treating symptoms.
The European Commission announced on Tuesday the approval of the antibody lecanemab to treat Alzheimer’s.
Lecanemab is intended for treatment in the early stages and is the first medication of its kind to be approved in the European Union, the commission said.
It is the first time the European Commission has authorized therapy which targets the underlying disease processes, rather than just the symptoms.
However, experts say that only a very small portion of Alzheimer’s patients are eligible for this therapy but follows a decision by the European Medicines Agency in November 2024 to recommend approval of the drug, reversing a previous decision.
Lecanemab is sold under the brand name Leqembi. The drug is already authorized in the United States, UK and Japan.
More to come…
DW News